Nurosym
Couldn't load pickup availability
Free shipping in 3-7 days
30 Day Money Back Guarantee
Certified Medical Device
4M+ Happy User Sessions
50+ Clinical Studies
Backed by 1,000+ Experts
Certified Medical Device
4M+ Happy User Sessions
50+ Clinical Studies
Backed by 1,000+ Experts
Nurosym harnesses advanced neuromodulation technology in a sleek and easy-to-use medical device. Validated by the world’s most elite research institutions in randomised placebo controlled scientific studies.
Nurosym sends patented electrical signals to the brain via the vagus nerve, activating specific therapeutic mechanisms that can counter dysregulation in the nervous system and significantly improve health outcomes, as proven by scientific studies.
Nurosym is the most studied CE-marked non-invasive vagal neuromodulation system in the world.
Get €70 for participation in our research study
Apply for studyWhat does Nurosym do
With Nurosym you can:
- Recover and activate your self-repair mechanisms by countering dysregulation in the nervous system.
- Build up your stress resilience, recover faster from stressful events and help prevent mood swings.
- Counter post-viral symptoms, like fatigue or brain fog.
- Maintain optimal HRV, recovery and reduce cardiovascular health risks.
- Relax and stabilize your mental state to help you fall asleep faster, achieve high-quality sleep, wake up with more energy and fewer negative thoughts.
- Improve digestive function by promoting healthy communication through the gut-brain axis.
- Reduce inflammation, pain and discomfort linked to your nervous system.
- Strengthen cognitive performance & focus to counteract the effects of aging.
How does Nurosym achieve all of this?
Vagus Nerve science is well established and Nurosym is a wearable device that is scientifically proven to modulate the vagus nerve via precise electrical impulses that are sent through the skin to a branch of the nerve on the outer ear that projects to the brain. Modern science has discovered that many persistent mental & physical symptoms are linked to a chronically dysregulated nervous system. The nervous system and one of its largest nerves - the vagus nerve - controls critical bodily functions, from breathing, heart rate, digestion to energy management, calmness and mood. When the vagus nerve is not functioning properly, symptoms can surface that relate to these processes (e.g. chronic tiredness or chronic stress). By signaling the nervous system in a natural way through modulating the activity of the vagus nerve, Nurosym helps to bring the body find back to its healthy state and manatin it in balance.
In this way Nurosym can support mental and physical health, helping alleviate symptoms arising from dysregulation in the nervous system.
Nurosym exerts its effects through various mechanisms:
- It triggers the “rest and digest” response, promoting relaxation, lowering heart rate and improving digestion.
- Reduces inflammation markers leading linked to pain & overactive immune response
- Decreases oxidative stress markers linked to chronic diseases and ageing.
- Increases neuroplasticity, rewiring the brain for better mood, better motor & cognitive learning.
Nurosym has been validated in 50+ completed scientific trials, third party tested and certified to the highest standard of devices that send signals to the vagus nerve. Nurosym was developed by Parasym, a world leading neurotechnology company, and uses Parasym’s proprietary Auricular Vagal Neuromodulation Therapy (AVNT)™ which has been studied in partnership with leading scientific research institutions including Harvard and UCLA.
Nurosym is the most scientifically validated device for targeting the vagus nerve via the ear available without prescription. It is the most studied neuromodulation system of this kind, widely considered as the gold standard by doctors and researchers.
Symptoms studied
Parasym’s AVNT technology has been scientifically studied in 50+ completed studies across a broad spectrum of populations and health and performance applications.
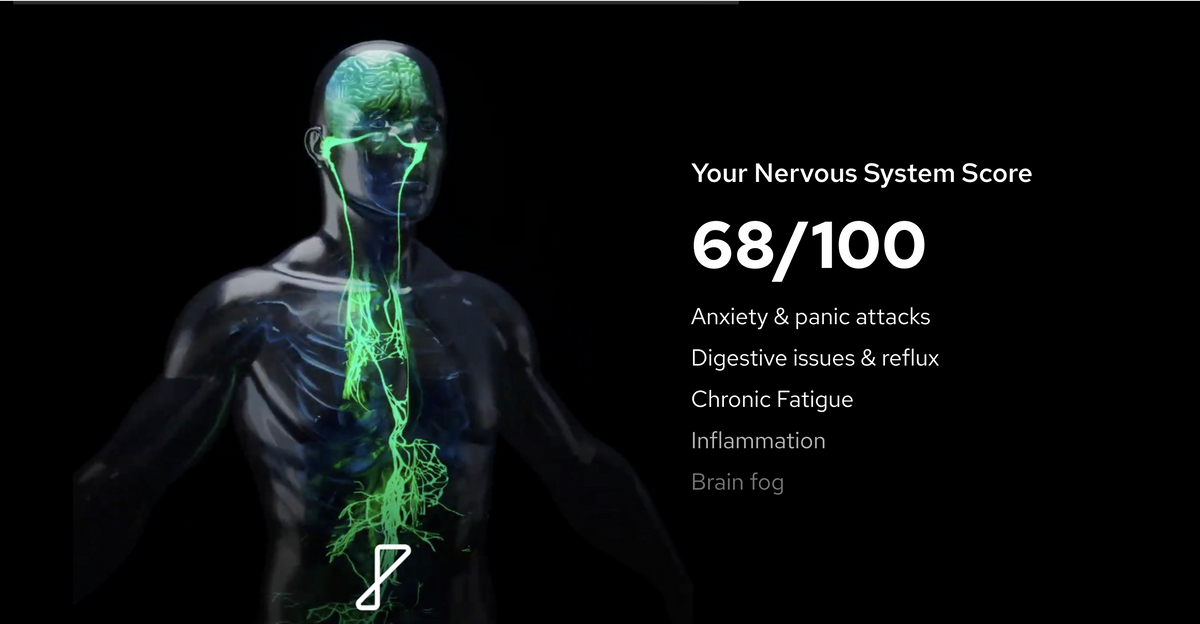
A focus of scientific studies has been on dysregulation of the autonomic nervous system. These symptoms have been linked to autonomic nervous system imbalance and vagus nerve impairment:
- Anxious states and stress related symptoms
- Chronic tiredness, fatigue, brain fog, burnout
- Digestive issues, irritable gut, reflux, overactive bowel, constipation, bloating
- Sleep problems
- Negative thoughts
- Inflammatory symptoms
- Pain, headaches
- Memory & attention problems
- Postural heart rate abnormalities or fainting
- Post-Viral Symptoms
- Widespread body pain
Our latest research data also suggests Nurosym improves learning, short-term memory and cognition.
Nurosym has been shown to promote relaxation, mood, sleep, healthy digestion, and increase HRV. Improved stress management alleviates symptoms of stress, burnout, and adrenal fatigue.
Parasym research is also investigating the impact of Auricular Vagal Neuromodulation Therapy on athletic performance, muscle recovery, weight management problems, substance dependency and more.
If you’re not sure if Nurosym is for you, consult with your health professional or contact support.
Who Is Nurosym For?
Nurosym is for adults looking to improve their health and performance.
Nurosym is not suitable for the following users:
- Users who have undergone surgery to cut the vagus nerve (cervical vagotomy);
- Users diagnosed with severe low resting heart rate;
- Users with a permanent (that cannot be removed before using Nurosym) implanted metallic or electronic device or jewelry at close proximity to the ear tragus;
- Pregnant women.
Below are listed situations that are also contraindications unless the user receives a different health opinion on the device use by their health professional and is under the supervision of a health professional while using the device. If you fall under one of these categories, your health professional might advise whether, in your specific case, the device is safe to use, potentially following certain precautions or under specific supervision:
- Users with any active implanted device (including electronic and/or wearable devices) e.g. cochlear implant, cerebral shunts, invasive vagus nerve stimulators, pacemakers or non-active but potentially interacting with the nervous system (e.g. metal implants);
- Users with known severe coronary disease or recent heart attack (within 5 years);
- Children users
- Pregnant women have not been evaluated in scientific studies.
- Both children users and users with pacemakers have been enrolled in scientific studies with Parasym technology, and no device related adverse events or interferences have been observed so far in the scientific setting.
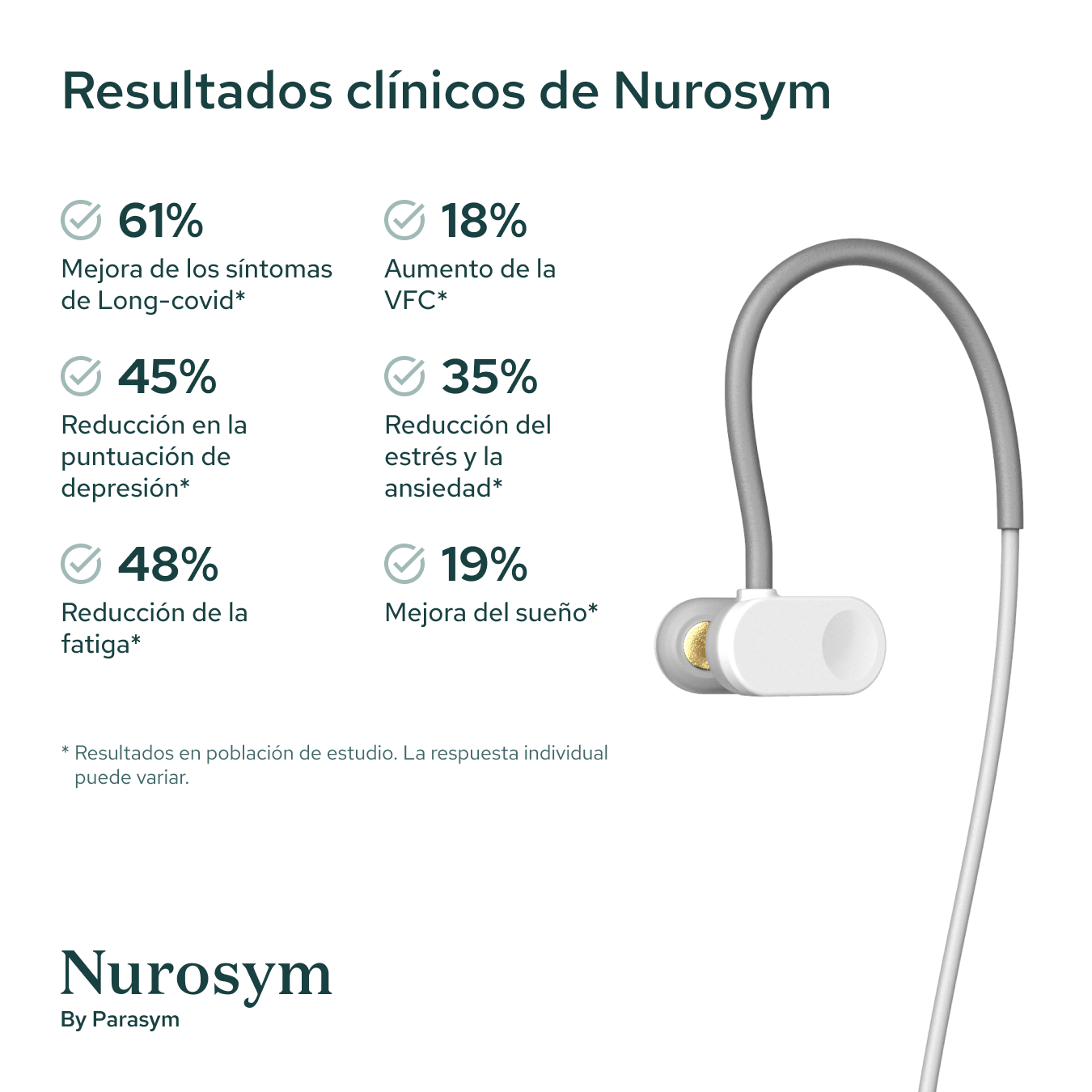
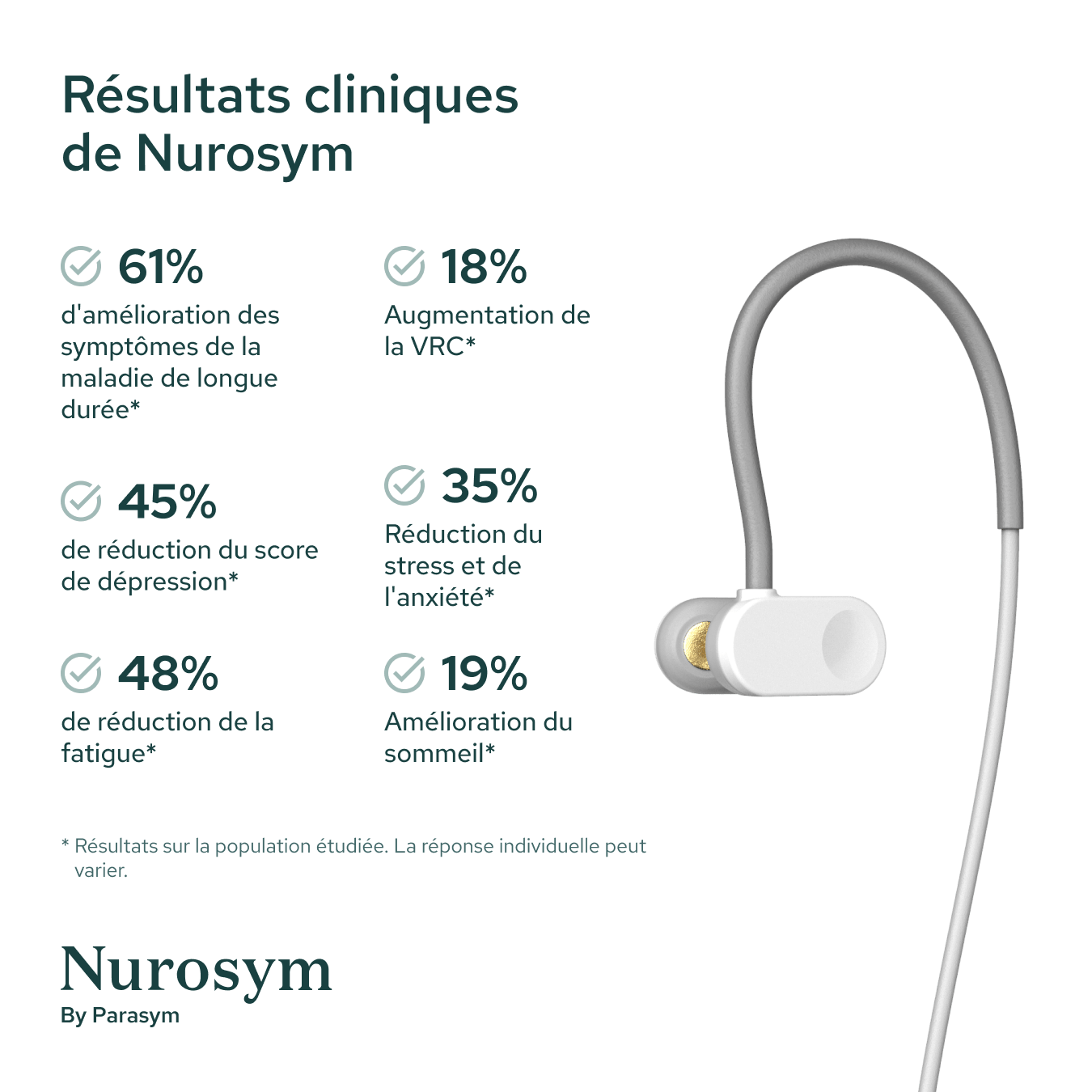
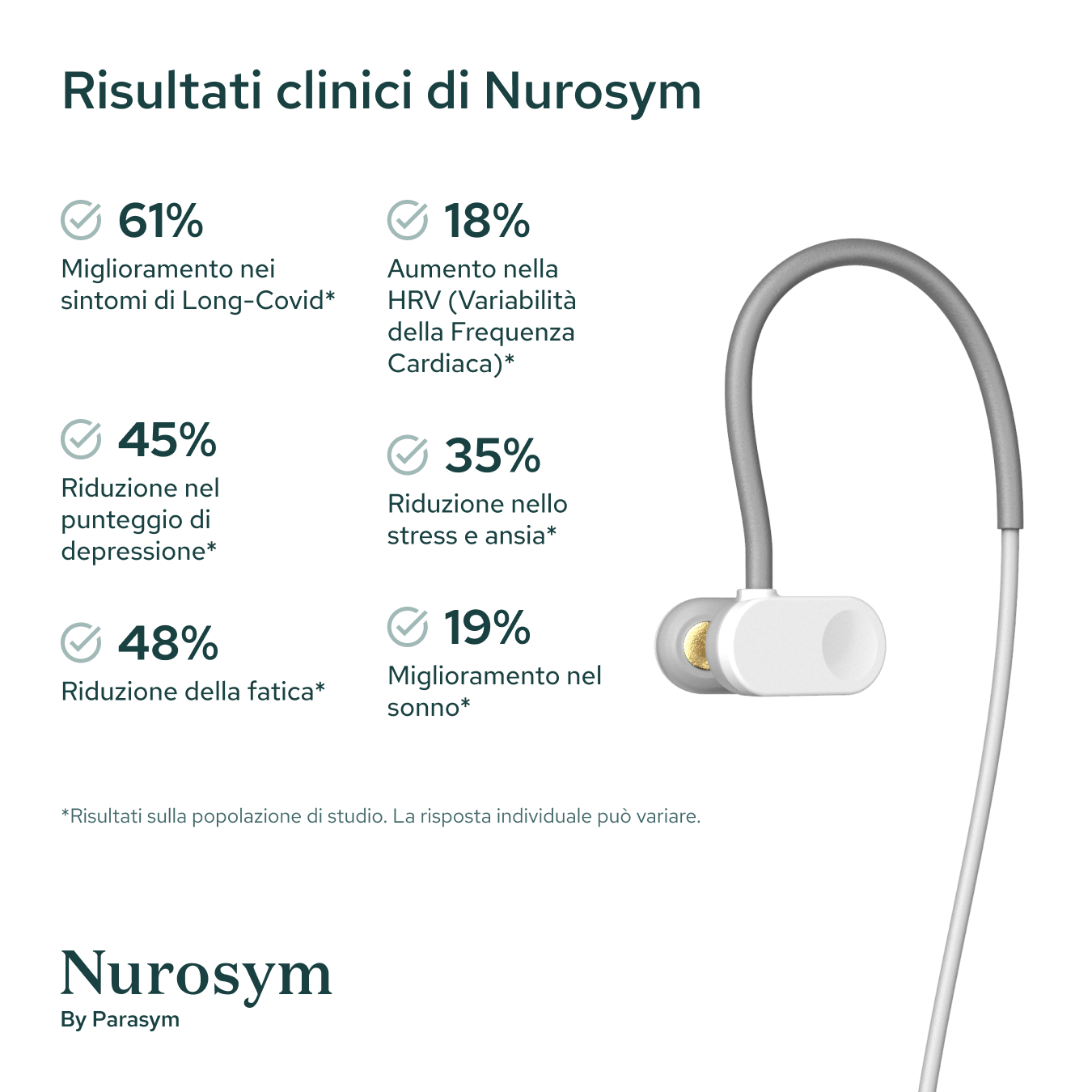
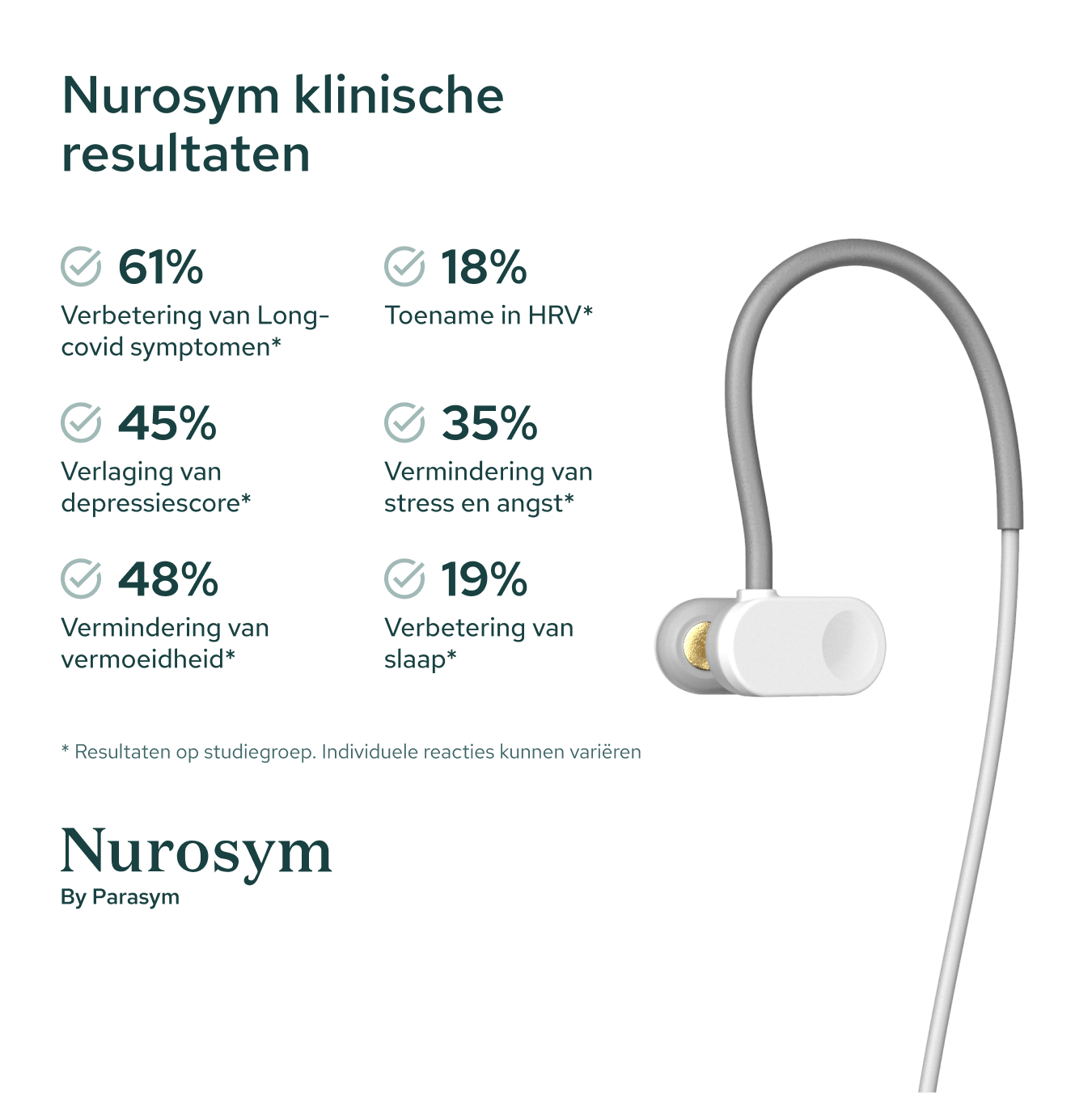
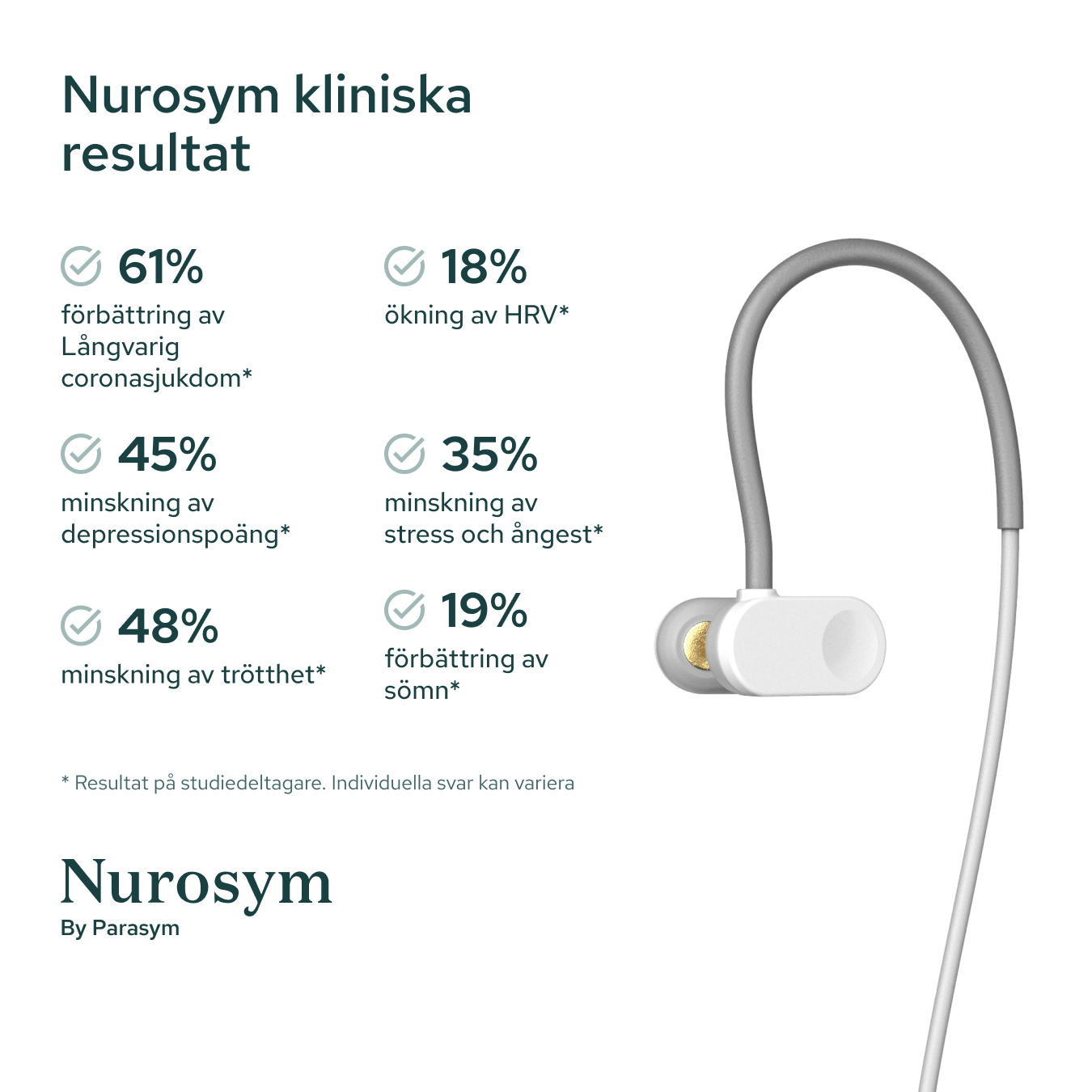
Results from scientific studies
Vagus Nerve Activity
Post Viral Symptoms
Fatigue
Sleep
Inflammation
Postural Heart Rate
Heart Rate Variability
Depressive symptoms
Oxidative Stress
Memory
Reading comprehension
Macrocirculation
Microcirculation
Attention
Anxious Thoughts
Palpitations
Heart Muscle Function
GI Symptoms
Blood Pressure
Results in specific study populations. Individual results may vary.
Science & Scientific Evidence
Nurosym has partnered with 100+ research institutions, including UCLA and Harvard, to conduct scientific studies to validate the safety and health benefits of Nurosym.
Our research is founded on these principles:
- Parasym’s studies are led by independent university experts, with no financial ties to us, unless explicitly mentioned.
- Our independent research ensures unbiased and genuine results.
- Independent transparency: All our study findings (positive or otherwise) are published and openly accessible to everyone.
- We collaborate with distinguished physicians and scientists, ensuring the pinnacle of research quality.
- A large part of our scientific evidence comes from Randomised Placebo-controlled Scientific Studies, which are considered the highest standard of evidence.
Getting started with Nurosym
How to use Nurosym in a few steps:
- Apply a small amount of moisture to the tragus.
- Place the earpiece on your left ear with the electrodes correctly on your left tragus (protruding part of your ear)
- Turn on your Nurosym
- Slowly increase energy level until you feel a light tingling sensation on your tragus.
- Nurosym is now active and delivering the signals to your parasympathetic nervous system.
- Notice the sensations of calmness, relaxation, decreased stress, improved mood and increased energy.
- Use Nurosym for sessions of 15-60 minutes and incorporate into your daily ritual.
Why trust Nurosym?
Gold standard in scientific research
- Nurosym By Parasym is a product of over $10M deployed in scientific research, 100+ partner research organisations including Harvard, UCLA, 50+ completed scientific trials evaluating Nurosym and publications in leading journals covering our exciting and scientifically significant results.
- This is why Nurosym is the world leading non-invasive vagal neuromodulation system used in scientific study, recommended by doctors and now widely adopted.
Developed in London, England, Scientifically Studied in the United States
- Nurosym has been independently scientifically studied in the United States by the worlds most elite scientific institutions like Harvard, UCLA, and the University of Pennsylvania, with scientific data published in some of the worlds most prestigious scientific journals like the Journal of the American College of Cardiology.
Independently scientifically studied in U.S.A, third party evaluated and certified
- Exposing your nervous system and brain to untested electrical currents can put you at risk of a permanent damage to the brainstem and vagal nerve fibres, worsening symptoms. Never use or suggest using electrical current devices on your nervous system that have not been designed for neuromodulation and scientifically studied to evaluate their effects on the body.
- Ensuring safety and efficacy requires extensive scientific studies that cost millions of dollars. This is why few Neuromodulation devices have supporting scientific trials showing they are both safe and effective.
- Beware of websites claiming to deliver benefits from uncertified therapies that present online reviews instead of scientific evidence. Carefully verify if a device you’re evaluating is from a reputable company in a trusted jurisdiction and has conducted scientific studies in the United States to evaluate use in this population.
- When considering any health intervention, be sure to evaluate the status of certification, scientific evidence, warnings and seek advice of trusted health care professionals.
Proven target activity
- In addition to safety, it is crucial to consider efficacy.
- The fact that a device is delivering a safe electrical signal to the vagus nerve and brainstem is not enough.
- Safe and effective neuromodulation can be thought of like a symphony, where the piano is your vagus nerve and the pianist playing the notes is the device. The precise notes need to be played at the right time and for the right lengths of time to have the result of the symphony.
- Without scientific data, we cannot know if a device is 1) actually sending the right signals to the vagus nerve 2) not sending signals that result in unintended neural pathway activation or supression.
- Our patented technology (AVNT) has been developed over 10 years of R&D, evaluated in 50+ completed scientific studies with a total cost of over $10M.
Efficacy confirmed in Randomised Placebo Controlled Studies
- The placebo effect can be a strong influencer on benefits of numerous devices and interventions. Only randomised placebo controlled scientific studies can demonstrate whether a device benefit is independent of the placebo effect. Make sure to verify quality of evidence provided.
- Nurosym has demonstrated benefits independent of placebo effects in rigorous Randomised Placebo Controlled Studies.
Reviews
Watch stories of recovery from users worldwide on our Nurosym Stories section.
Certifications & Safety
Nurosym is the most scientifically studied device of this kind and considered safe when used as intended. Most users encounter no issues and the most commonly reported issue with using Nurosym is a mild irritation on the skin where the electrodes are placed, however this is rare and typically resolves after ceasing use.
Nurosym has been evaluated in rigorous independent scientific studies and shown excellent safety profiles. The technology powering Nurosym, AVNT (Auricular Vagal Neuromodulation Therapy), has been certified to the highest standard of devices that send electrical signals to the vagus nerve by third party laboratories.
AVNT has now been used widely with over 4M+ user sessions and 0 serious adverse events reported in studies to date.
If you are unsure whether Nurosym is right for you, be sure to contact your health care provider.
Shipping & Returns
- Ships from California U.S.A.
- Ships to U.S.A., EU, UK, and select global locations.
- Orders processed within 24hrs.
- No import fees for U.S. customers. Import fees may apply outside the U.S.
- 30-Day Money-Back Guarantee – Return within 37 days if unsatisfied.
30-Day Money Back Guarantee
If you try Nurosym for 30 days and aren’t happy with your results, we’ll send you a full refund of your Nurosym Kit. No questions asked.
Given the remarkable results Nurosym has had in scientific studies and with our users around the world, we’re confident in its health-restoring effects.
This refund period is available 30 days after the date of purchase and expires the following week (37 days after the date of purchase).
To receive a refund, please contact support here: care@nurosym.com.
Questions?
Not sure Nurosym is for you? Contact our support team at care@nurosym.com
Disclaimer
Important safety information
Nurosym might not be right for you. Before ordering, be sure to read about intended use, warnings, precautions and contraindications.
Consult with your health professional before using any medical intervention.





































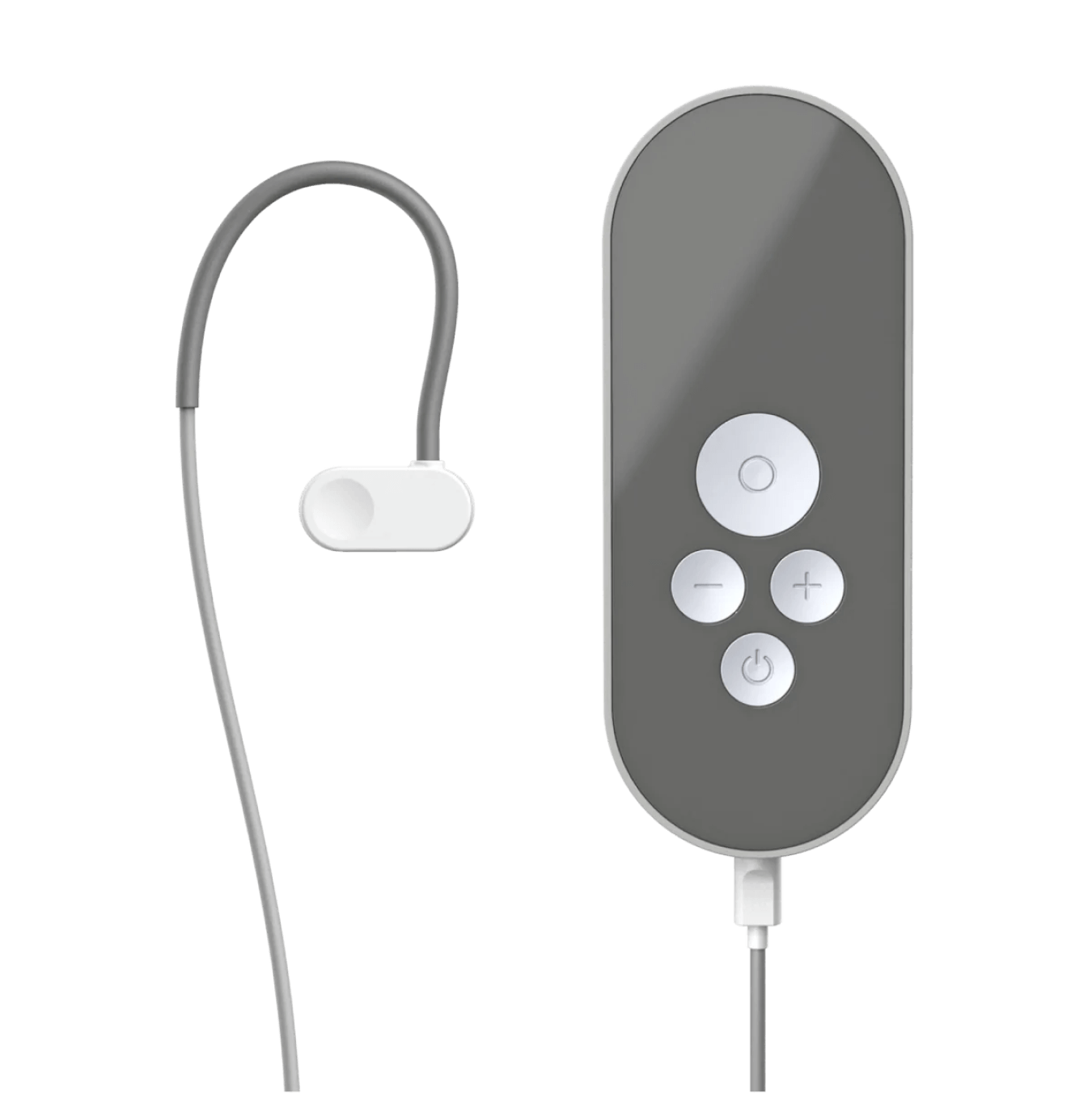




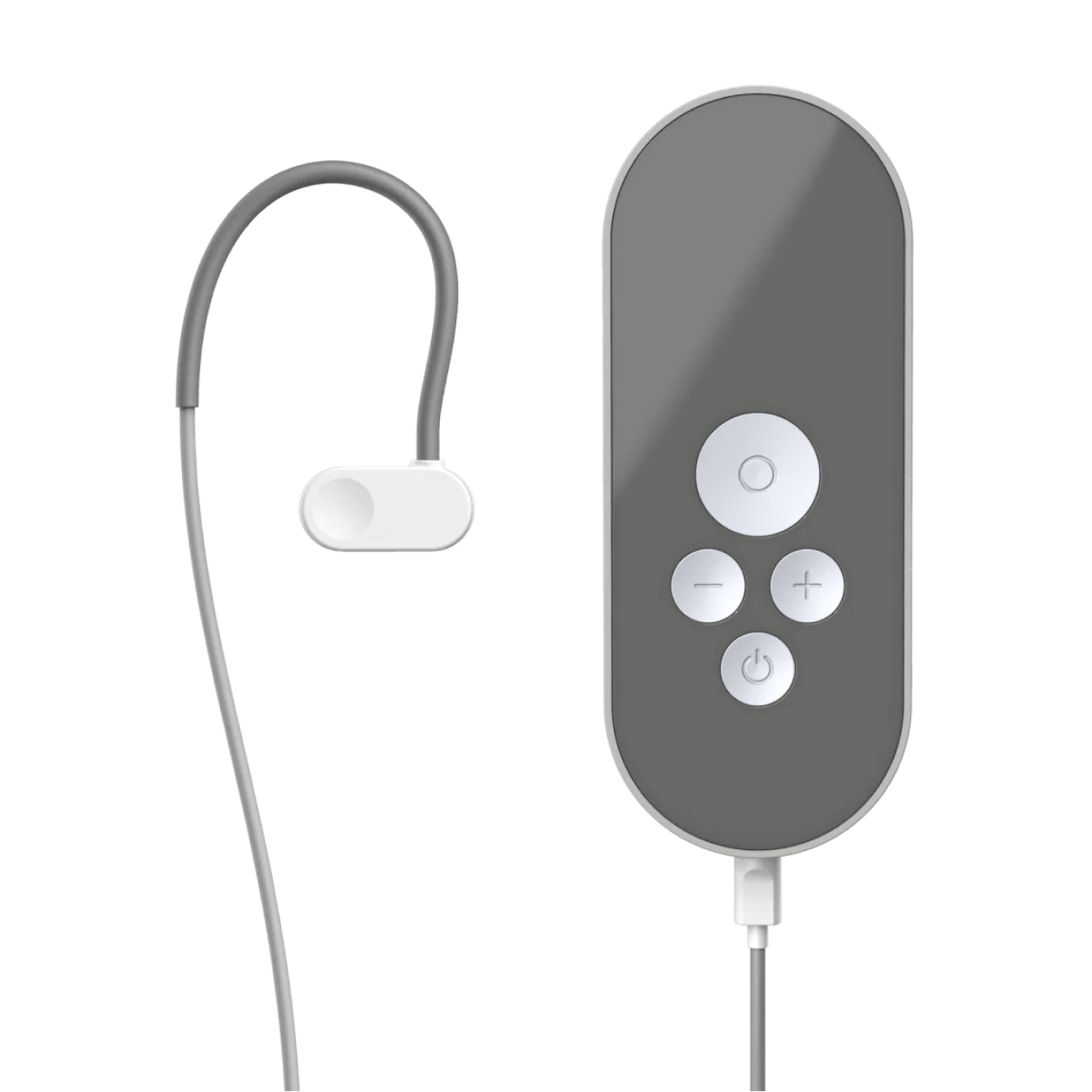




How to use Nurosym

Attach the earpiece and wear just like a headphone
Position the cable over and behind your ear to keep the earpiece in place
Activate Nurosym
When Nurosym is active you will feel a pleasant tingling on your tragus
Use for 30-60 minutes per day
Seamlessly integrate with non-intensive daily activities
Experience the benefits of using Nurosym
Learn more about benefitsHow Nurosym is helping our community
Read More Stories
“Nurosym seems to have had a positive impact on me. I am happy to say that the intervention seems to have been effective.”
James Gill
Doctor
“I have been very pleased with my Nurosym. Before receiving it, I had not been going out much and had not driven for months. Within a few weeks, I started going out for coffee with a friend, managing more around the house, and even driving again locally. A further benefit is improved sleep.
When I bought this wearable device, I did not know what benefits to expect and it exceeded my expectations. I would happily recommend it to anyone with a diagnosed vagus nerve disorder and depressive states or anxious thoughts.”
Pamela
“With Nurosym, I reach my best scores every day. My HRV increased from around 120 to 150–155, and my sleep quality improved.”
Tanya Boychuk
Professional football player
“I have chronic tiredness and post-viral fatigue, which led to persistent depressive states, impulsivity, and high stress. I have been using the Nurosym wearable device daily for several months and felt significant improvements in my symptoms.
The most noticeable change was a major drop in stress levels. My sleep quality, heart rate variability, and resting heart rate also improved. I also noticed an improvement in my mood. While using the device, I usually combine it with breathing exercises to further support calming the nervous system.”
Luisa Hermann
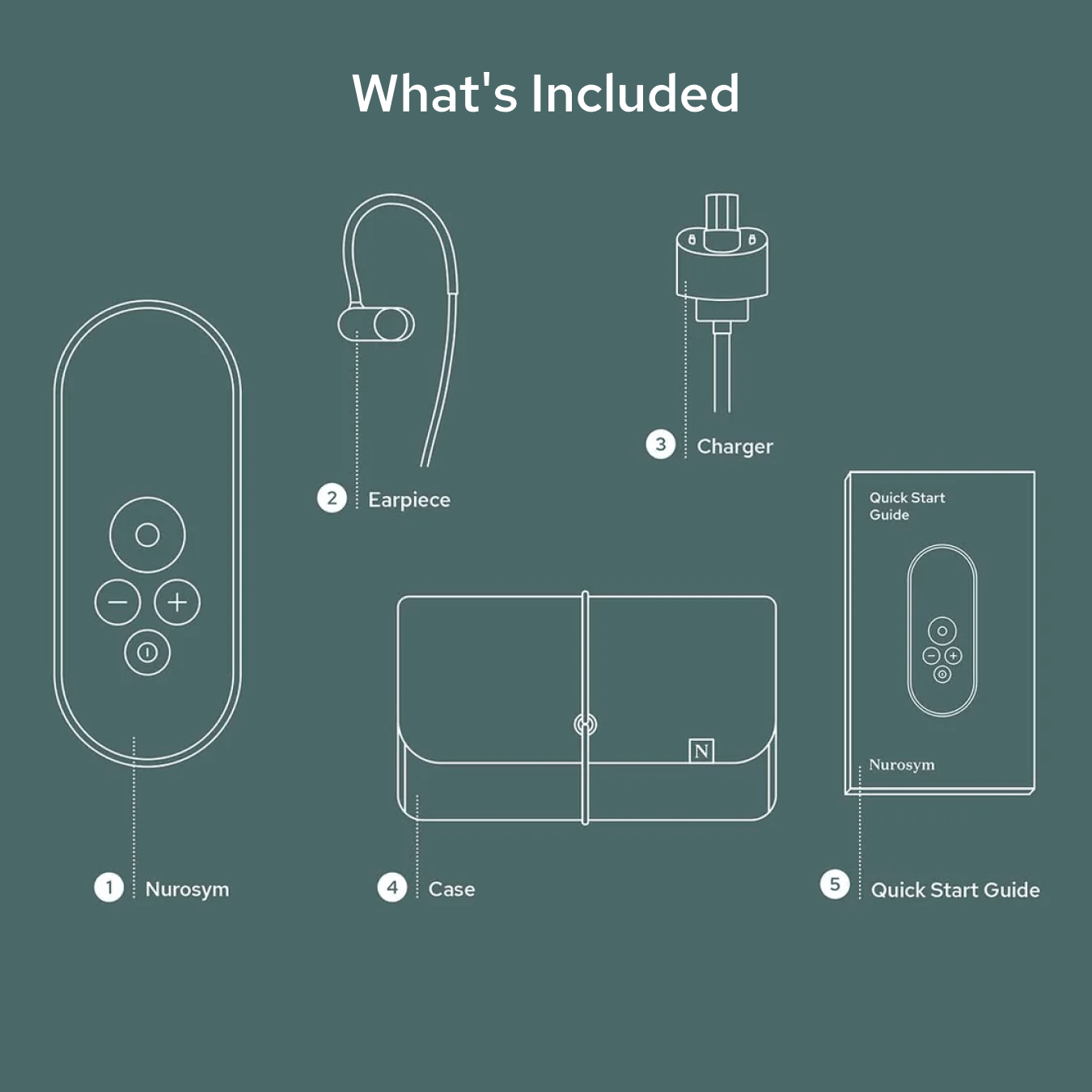
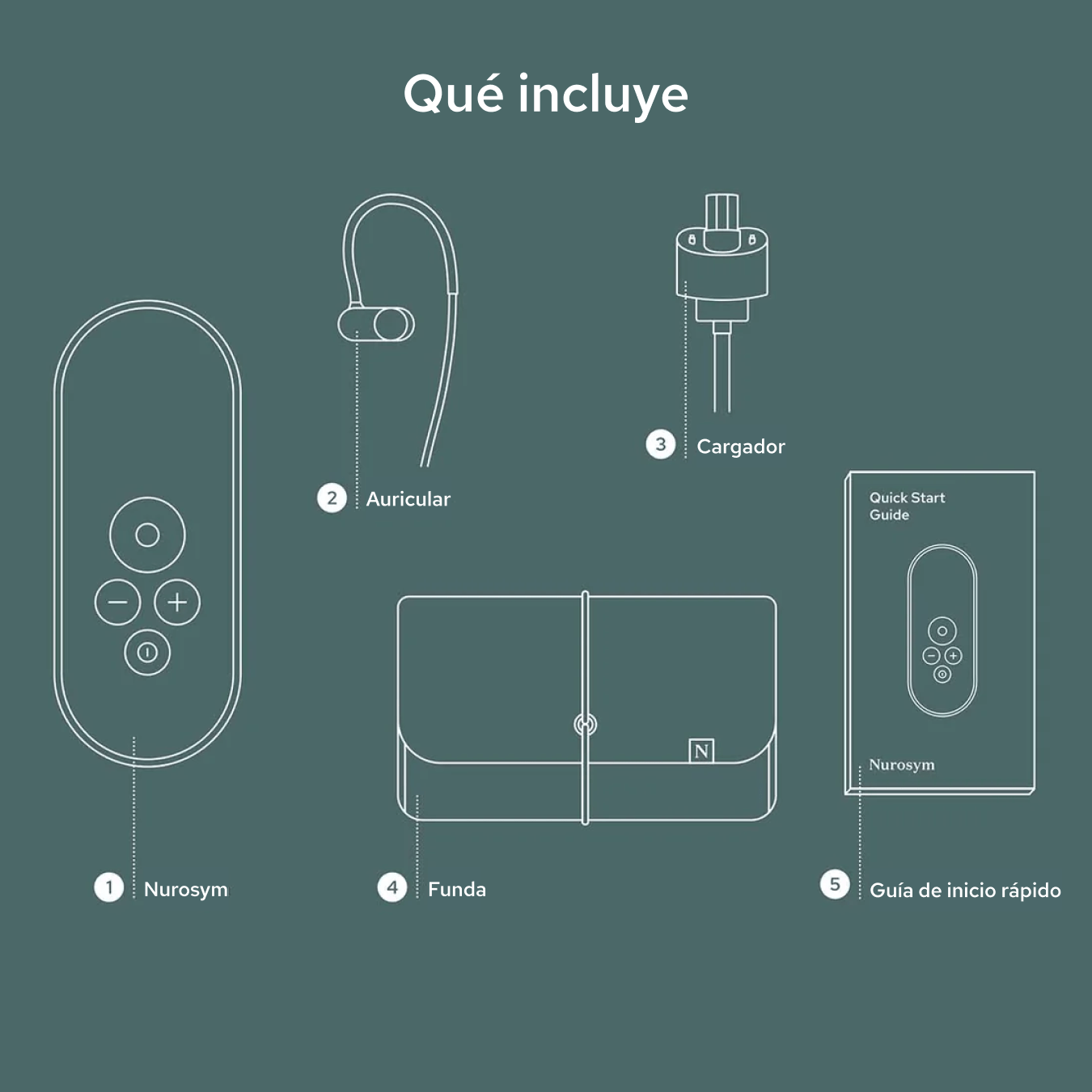
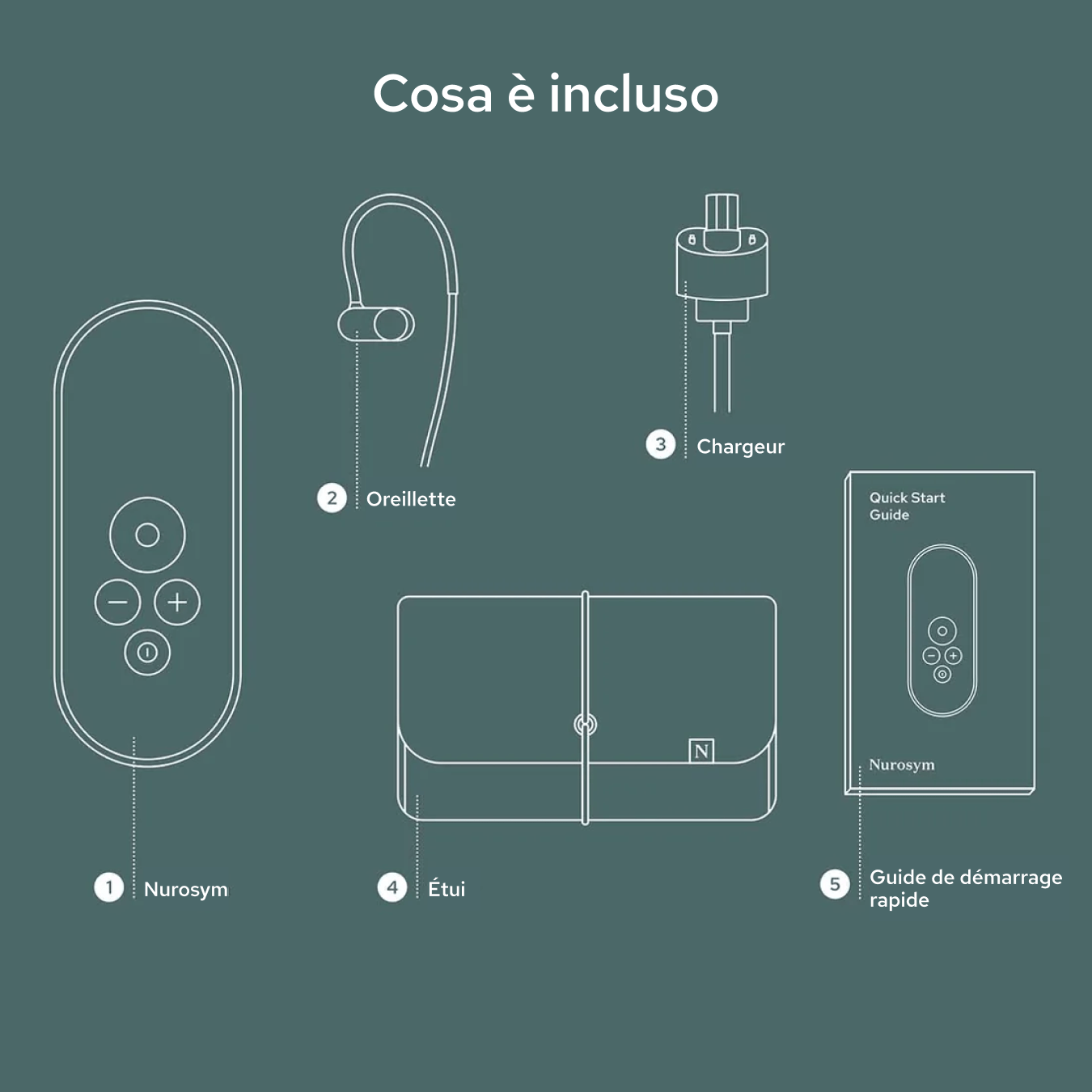
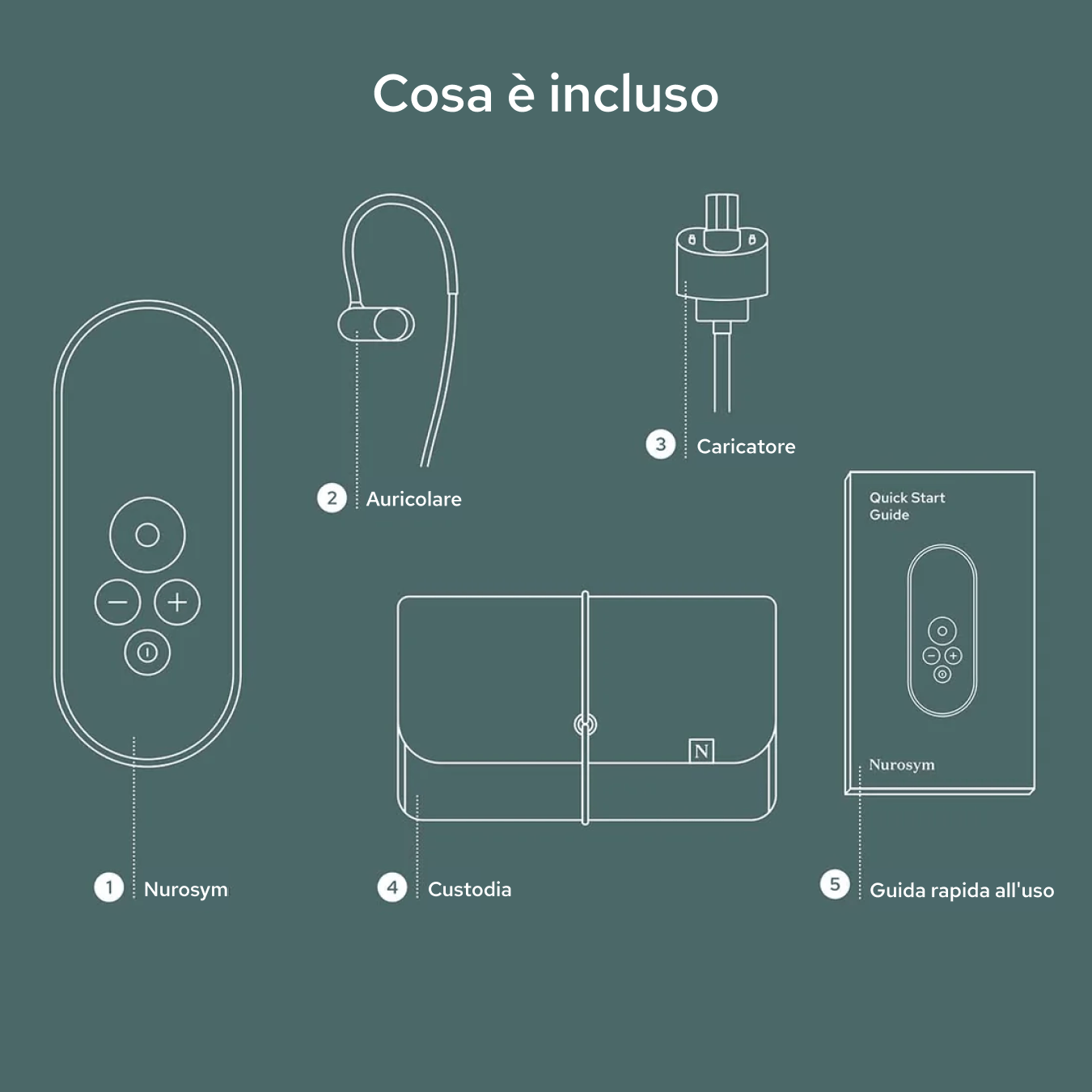
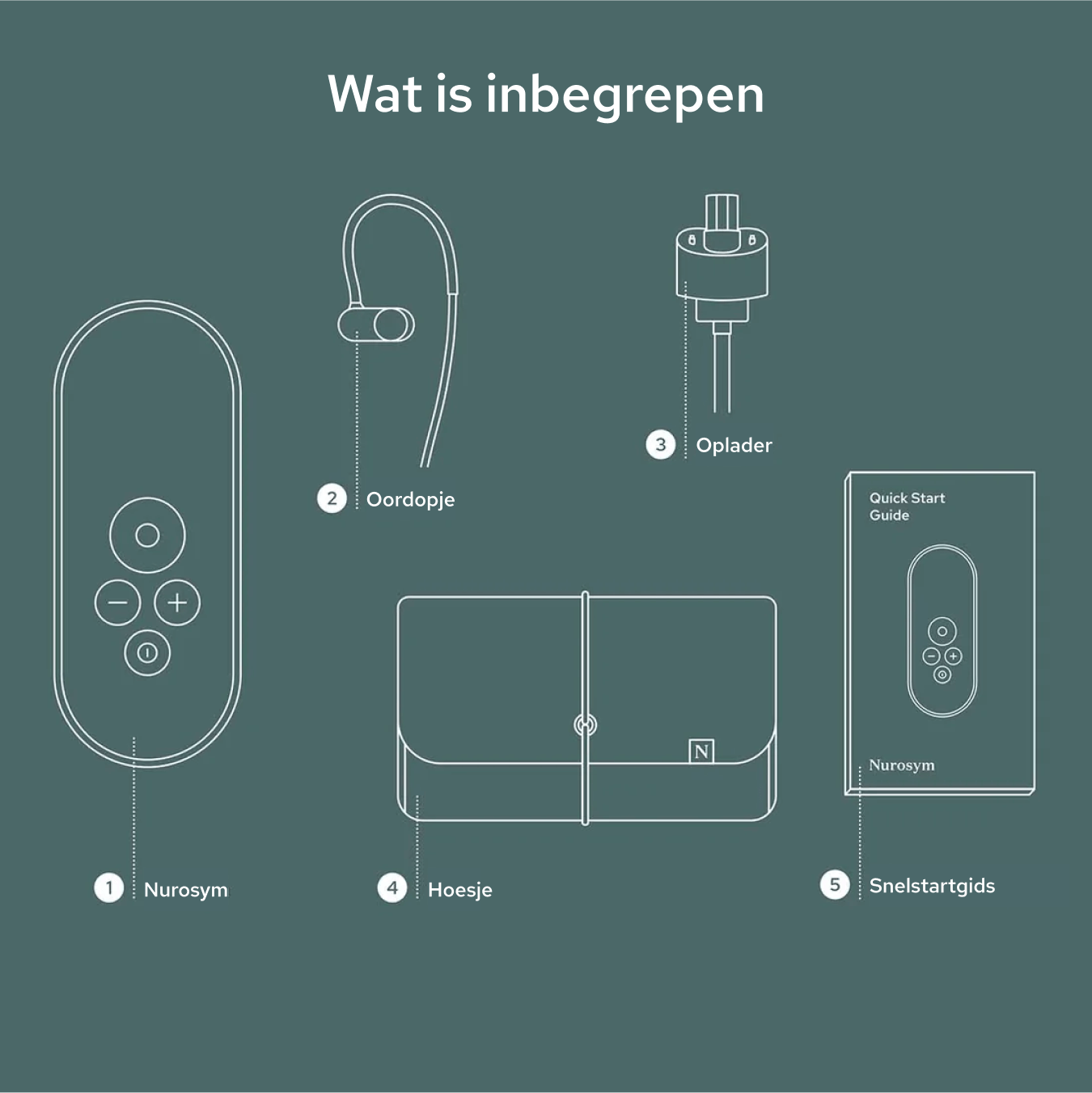
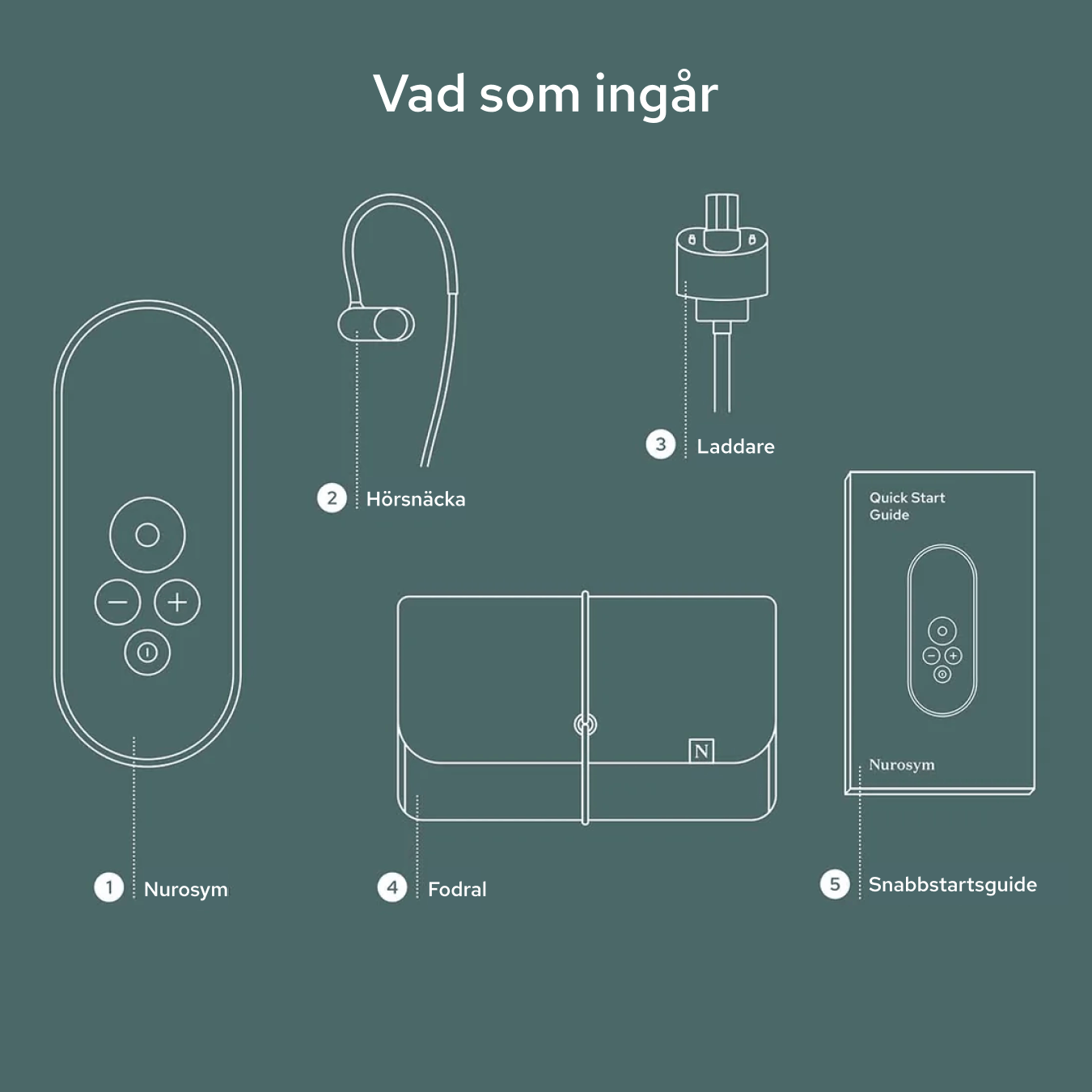
Unboxing your Nurosym
Your Nurosym kit includes the Nurosym device, a comfortable earpiece, a charger, a Quick Start Guide for easy setup, and a protective case for secure storage and travel.
Nurosym
Earpiece
Charger
Case
Quick start guide
Inspired by you, certified by science
Experience the Future of Bioelectric Medicine
Claim €70 OFF the most studied vagal neuromodulation device in the world
Take part in our mission to advance research in neuromodulation and claim €70 rebate on your device upon completing the study.
* Takes less than 2 minutes to complete.