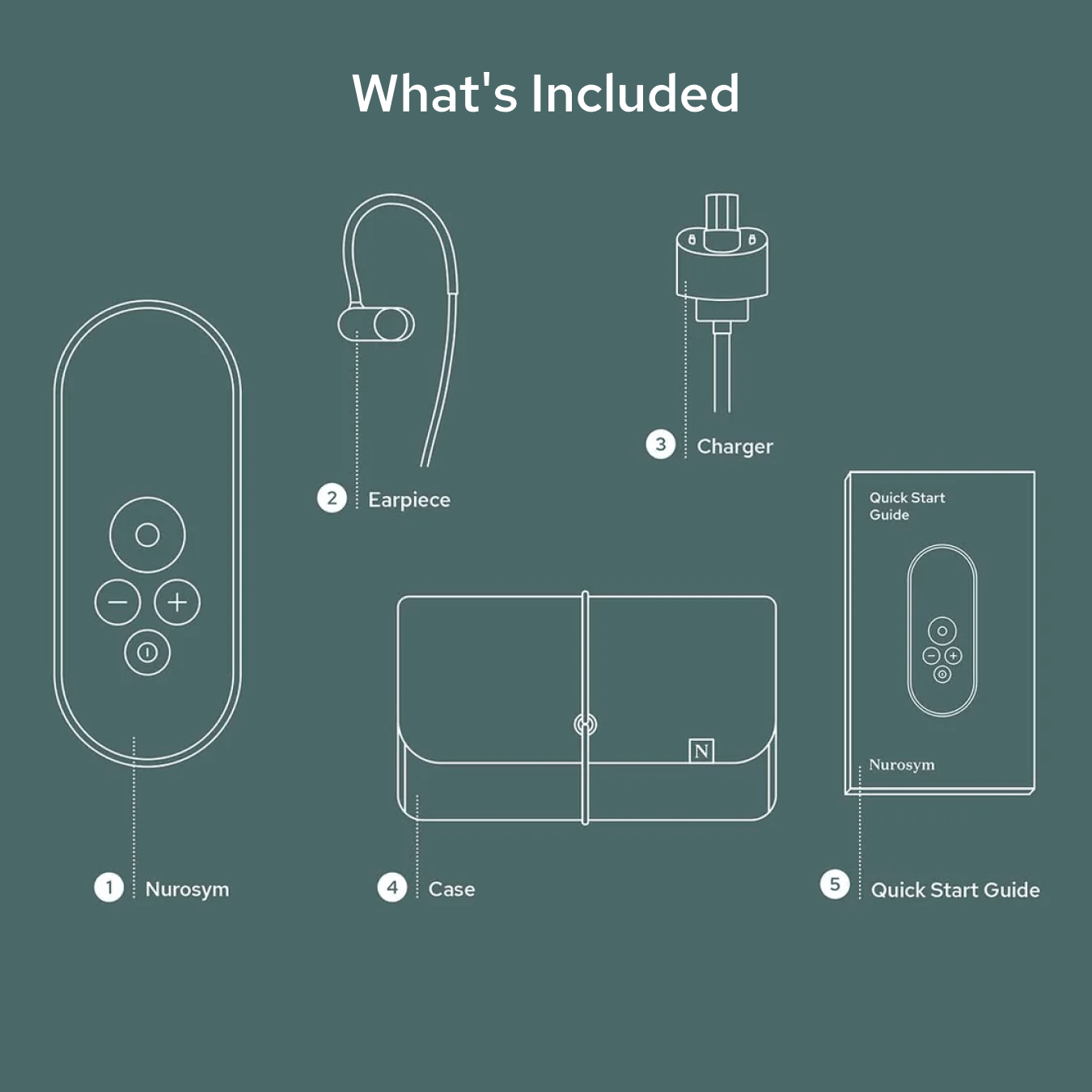
How to use Nurosym
Attach the earpiece and wear just like a headphone

Activate Nurosym

Use for 30-60 minutes per day

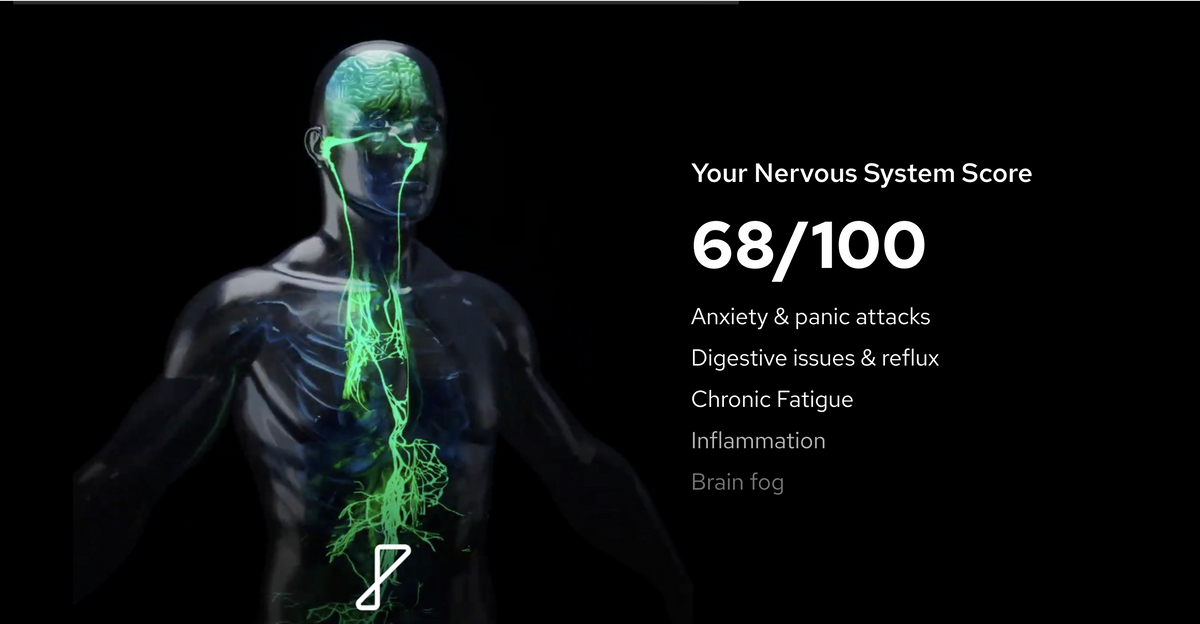
Experience the benefits of using Nurosym

How Nurosym is helping our community
"Nurosym seems to have had a positive impact on myself".
"I am happy to say that the intervention seems to have been effective".
Stress decrease: -44%
HRV increase: +68%
James Gill
Doctor
"Now with Nurosym, I'm able to get to my best scores every single day".
"My HRV used to be 120, and now it sits at around 150-155".
"...really helped with my sleep performance… I'm able to relax and get to the state I need to be to have a good night's sleep..."
Tanya Boychuk
Professional football player
Nurosym Stories
Read more stories"I have been very pleased with my Nurosym. I had not been going out much and had not driven for some months when I received it. Within a few weeks, I had started going out for coffee with a friend and managing to do a little more around the house. Best of all, I have even started driving again locally. A further benefit is improved sleep.
When I bought this wearable device, I did not know what benefits to expect and it has exceeded my expectations. I would happily recommend it to anyone with a diagnosed vagus nerve disorder and depressive states/anxious thoughts. I wish it had been available before the health professionals started throwing medication at me, which I am not able to tolerate."
Pamela
“I have got chronic tiredness, Post-Viral Fatigue, and because of that, I got persistent depressive states/low mood. In addition, I am very impulsive and often stressed. Since many months, I am now using the Nurosym wearable device on a daily basis. I felt a significant improvement in many of my health symptoms. The most significant improvement I realized is my stress level – it dropped significantly.
The quality of my sleep also improved. My Heart Rate Variability and my Heart Rate in rest also improved. I think my mood also improved during the use. While I am using the device, I do usually also breathing exercises to further support the parasympathetic nervous system to calm down.”
Luisa Hermann

Inspired by you, certified by science
Poor health outcomes and a lack of options motivated us to innovate - developing technology which can be used from the comfort of home without the problems of pharmaceuticals. Nurosym is a certified and patented wearable device.